Peptide API Services
- Select
- Select
Home - Services & Solutions - Peptide - Development & Manufacturing - API
Changzhou Site, China
74 acres
Taixing Site, China
168 acres (Phase I: 84 acres)
Our peptide API process development and manufacturing platform provides an end-to-end solution from preclinical to commercial stages. We have built industry-leading capabilities and capacities to support your needs across all development stages, ensuring knowledge retention throughout the product lifecycle. The total reactor volume for Solid Phase Peptide Synthesis (SPPS) is over 100,000 L.
Comprehensive Peptide Process Development Capability
Our highly experienced peptide development teams, comprised of several hundred scientists, support the process development of various peptide modalities, unnatural amino acid derivatives and complex peptide conjugates from preclinical to commercial production.
Our services include, but not limited to:
- Long peptide
- Cyclic peptide
- Complex peptide, such as long peptides via chemical ligation, PNAs (Peptide Nucleic Acid), peptoids, peptidomimetic peptides, branched peptides and dendrimers
- Peptide conjugates, such as Peptide Drug Conjugate (PDC), Radionuclide Drug Conjugate (RDC) and Peptide Oligonucleotide Conjugate (POC)
We built a comprehensive process technology tool box to accelerate your peptide development across the product life cycle.
- Solid phase peptide synthesis, liquid phase peptide synthesis and hybrid approach
- Linear synthesis and convergent synthesis
- Continuous chromatography
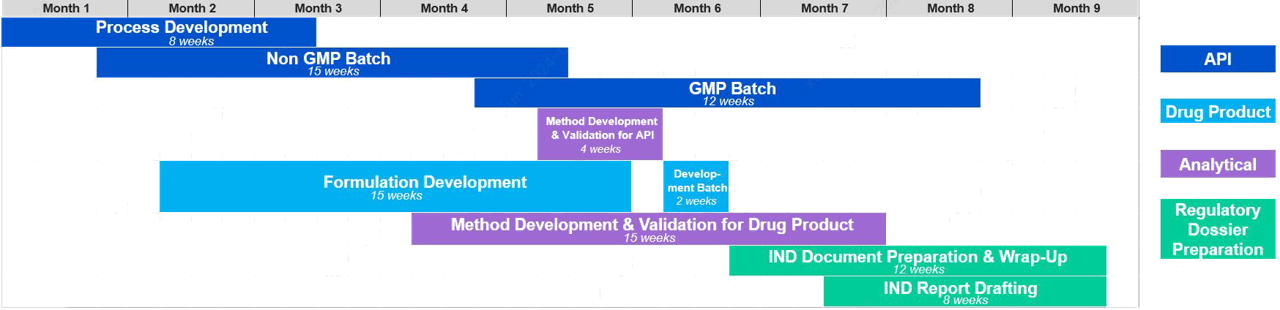
Case Study: Integrated CMC Solution for IND Application in 9 Months
We supported the 30+ AA macrocyclic peptide project by providing integrated CMC services, including API process development, manufacturing, formulation development, and CMC writing. We successfully completed the IND application within nine months, overcoming the challenges of an original route yield of less than 1% and a limited supply of a special building block for conjugation.
In the end, we developed a new route based on SPPS, and synthesize the main-chain and side-chain in parallel; we further achieved cyclization on resin, and completed the API synthesis via solid phase coupling strategy, which increased the yield by fourfold. The conjugation process was optimized to facilitate Tox and GMP batches in a more efficient manner. In-parallel and seamless workstreams allowed the entire project to be completed in nine months.
Manufacturing
With our global standard quality system, we are well positioned to be your strategic manufacturing partner for reliable and robust supplies of your peptide APIs, from gram to metric ton scales.
Our custom synthesizers and cleavage systems are designed to minimize risks of both microbial contamination and cross-contamination. Our peptide platform ensures knowledge retention and scale-up throughout your product life cycle, while expediting your development through seamless collaboration among different functional teams.
At our Changzhou and Taixing sites in China, we have over 20 Solid Phase Peptide Synthesis (SPPS) production lines with a wide range of synthesizers from 20 L to 5,000 L. The total reactor volume for SPPS is over 100,000 L. And we are still expanding our SPPS capacity at Taixing and Singapore sites in the next few years.
Our peptide manufacturing platform is also equipped with the latest technologies, including Liquid Phase Peptide Synthesis (LPPS), reactor-in-series (with PAT data collection) , continues flow chromatography, Tangential Flow Filtration (TFF) / precipitation, etc.
Liquid Phase Peptide Synthesis (LPPS)
- Route design and protection strategy selection for a wide range of peptides
- Quality by design (QbD) to support LPPS process performance qualification
- Dedicated crystallization process team, avoiding most of the prep-HPLC separation
- LPPS and SPPS combined approach for complex peptides synthesis
- Both batch mode and flow mode capable
- Hydrogenation capable

Peptide Conjugate
Peptide conjugation is a rapidly expanding field, serving as a new modality for targeted drug delivery. This technique enhances efficacy and reduces side effects in cancer treatment. Our comprehensive peptide platform, combined with our small molecule chemistry capability, provides peptide conjugate development and manufacturing.
We support various peptide conjugates including but not limited to:
- Peptide-PMO
- Peptide-Oligonucleotide
- Peptide-Toxin
- Peptide-Metal Complex
- Radionuclide Drug Conjugate (RDC)
- Peptide-GalNAc, etc
Our services includes:
- Design linkers with optimal size, suitable release mechanism and compatible conjugation chemistry
- Develop payload derivatives with modified toxicity and/or special linkage site
- Synthesize customized drug-linker complexes
- Seamless scale up from discovery to commercial

Samples of 350+ Linkers
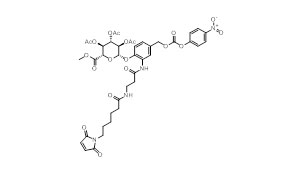
Samples of 40+ Payload Modifications
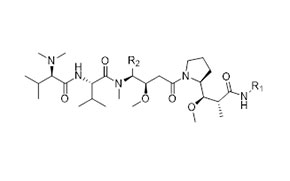
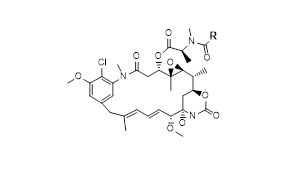
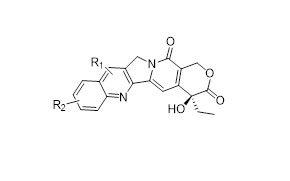
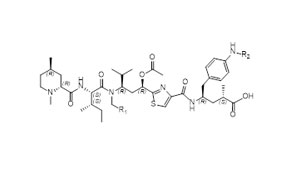
Case Study: Our Integrated Peptide-toxin Development and Manufacturing Service in 9 months
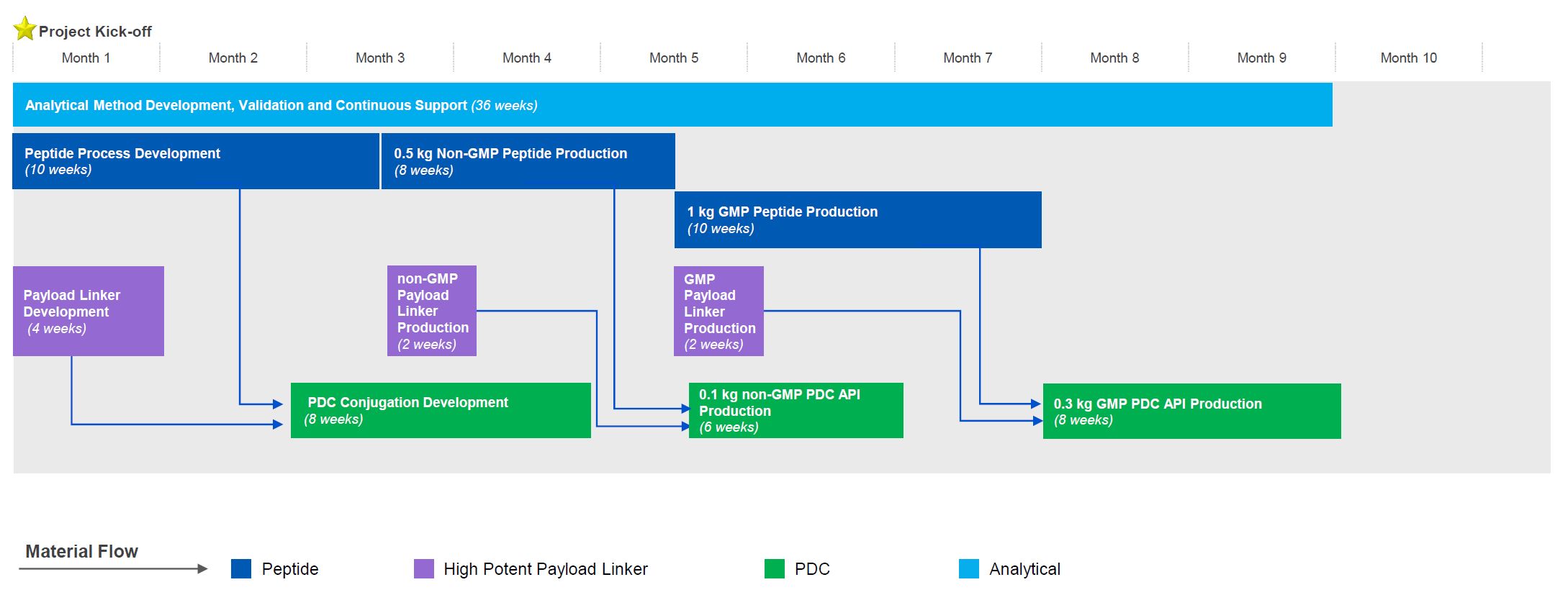
- Seamless collaboration with in-parallel activities across 4 workstreams
- Swift sample transport and information sharing from upstream to downstream teams
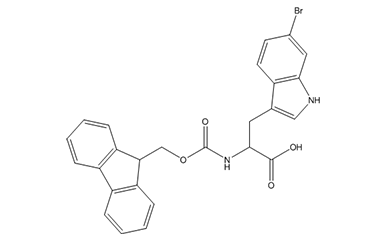
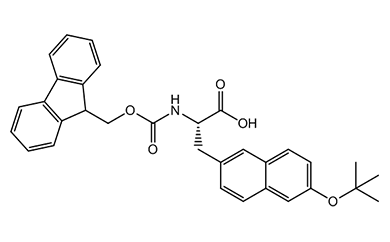
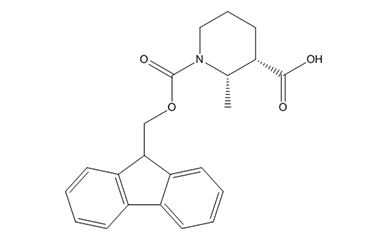
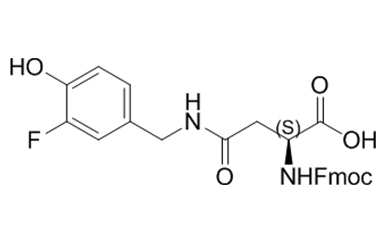
Unnatural Amino Acid
We offer over 2,500 catalog products of unnatural amino acid, including backbone modified / side chain modified amino acid and derivative of natural amino acid, etc. We delivered 12,000+ unnatural amino acids in last 5 years.
Visit Amino Acid e-Shop
We are experts in producing unnatural amino acid catalog products, including the most complex structures, tailored to our customers’ specifications from kilo to metric ton scales. Upholding global quality standards, we develop robust processes for amino acid products, ensuring superior quality.
Experience Highlights
α-Me-amino acids:
- We utilize the natural amino acid Alanine to design α-Me-amino acid derivatives. This methodology can be extended to other natural amino acids, such as Leucine, Phenylalanine, etc.
N-Me-amino acids:
- We have developed a cyclization process that allows methylation to be achieved in just two steps.
PG series:
- All protecting strategies, including Boc, Fmoc, Cbz, Mtt, DDE, and IVDDE, can be implemented.
α- and β- construction series:
- We have developed enzyme catalyst techniques to construct selectable α- or β-site unnatural amino acids.
NCA series:
- We have developed a process to effectively control the quality of NCAs.
Related Resources
Empowering Highly Potent Peptide Drug Conjugate Scale-up with Flow Chemistry






