Peptide Analytical
- Select
- Select
Home - Services & Solutions - Peptide - Development & Manufacturing - Analytical
WuXi TIDES has a dedicated analytical team with extensive experience and expertise in peptide analysis across all development phases from pre-clinical development to commercial manufacturing, covering both drug substance and drug product.
Equipped with state-of-the-art instruments and co-located with the API process development team, our peptide analytical service streamlines your peptide development process with a customized, flexible, and integrated analytical solution.
Analytical Development
Drug Substance Specific Analytical Services
- Analytical development for drug substance specific methods (purity, sequencing) and feasibility for peptide generic methods
- Forced degradation study with impurity qualification and quantitation
- Release testing and stability study under R&D conditions
- Reference standard and impurity marker qualification and retesting
- Physicochemical property study and structure elucidation
- Microbiological safety: endotoxin limit and quantitation testing, bioburden for microbial limit test, microbe ID and strain typing
Drug Product Specific Analytical Services
- Container-Closure Integrity Testing (CCIT), including quantitative testing such as vacuum decay method, high-voltage leak detection and qualitative testing such as microbial challenges, tracer liquid, etc.
- Foreign and particulate matter observe in situ, verifying by flow imaging (MFI) and identifying via IR/Raman/EDX, osmolality
- Purity by LC-MS, including ID and quantitation utilizing Q-TOF HRMS and quadrupole LC-MS specifically for hard-to-resolve parenteral impurity analysis

Peptide Enantiomeric Purity Test
Determining the enantiomeric purity of peptides accurately can be challenging. Peptides with different amino acid isomers share the same physicochemical properties, making them difficult to analyze intact. Free amino acids do not retain well for HPLC-UV analysis, and peptides containing multiple amino acids can interfere with each other during analysis. Despite these challenges, we have the capability to analyze the enantiomeric purity of all 19 isomeric natural amino acids (Asparagine and Aspartic acid, Glutamine and Glutamic acid are reported together), as well as Ornithine.

Quality Control
- Method qualification and validation (IND/NDA, commercial)
- Transfer GMP validated methods according to USP <1224>
- Full release testing (raw materials, reagents, intermediates, drug substance and drug product)
- Accelerated and long-term stability studies under ICH compliance
Special Capabilities
- Process related impurity identification: 2D UPLC coupled with Q-TOF HRMS
- Identification by tandem LC-MS/MS for Sequence Confirmation
- Amino acid analysis and enantiomeric purity: Acid hydrolysis and derivatization followed by LC-MS analysis to accurately evaluate the amino acid ratio and D-isomer impurity amount in peptide sequence
- Identity by peptide mapping: Enzymatic digestion followed by LC-MS analysis for characterization and confirmation of peptide drug substance

Other Equipment
- Amino Acid Analysis (AAA)
- Elemental Analysis (CHN)
- Enantiomeric Purity
- Polarimetry
- Refractometry
- Titration
- Turbidimetry
- Osmolality
- Total Organic Carbon (TOC)
- Total Viable Aerobic Counts
- Endotoxins (LAL-test)
- Heavy Metals
- Sulfated Ash
- Residue on Ignition
- Polydispersity

Key Equipment
- Chromatography: UPLC, HPLC, GC, IC, 2D-UPLC, HPLC-CAD
- Mass Spectrometry: Q-TOF, MALDI-TOF, LC-Orbitrap, LC-MS, LC-QQQ, 2D LC-MS, GC-MS
- Physicochemical Testing: FT-IR, UV-Vis XRPD, DVS, DSC, TGA, polarimetry, 400/600 MHz NMR, CD, polydispersity
- Elemental Analysis: ICP-OES, ICP-MS, elemental analysis (CHN), Kjeldahl analysis
Case Study 1: Assessing Peptide Main Peak Purity by 2D-UPLC-TOF
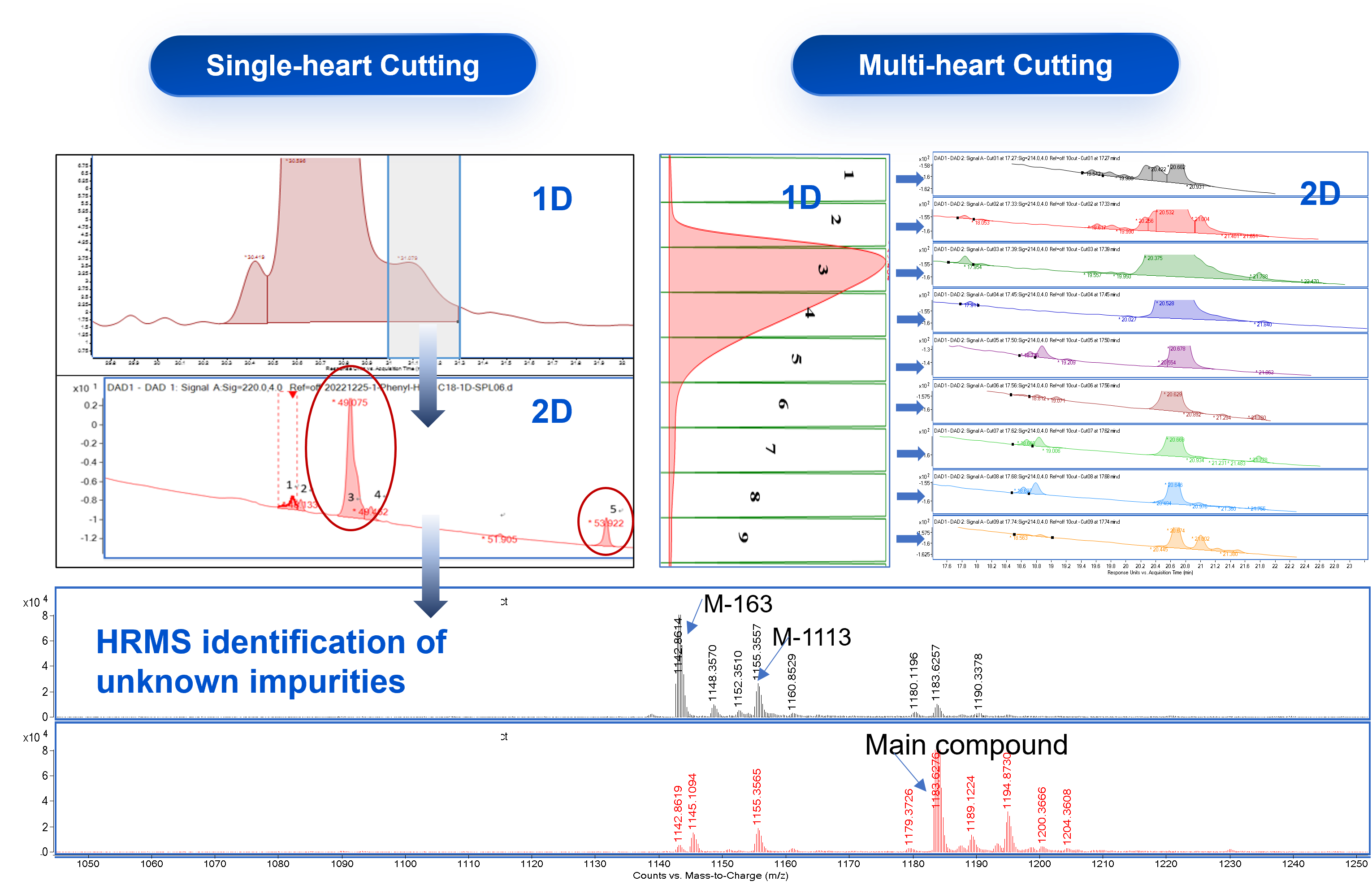
Multi-heart cutting 2D-UPLC-TOF is routinely used for the quantitation and qualificaiton of impurites that prove challenging to be separated by regular RP-HPLC, which greatly increases the efficiency for the impurity analysis and accuracy of data.
Case Study 2: Sequencing of Cyclic Peptide
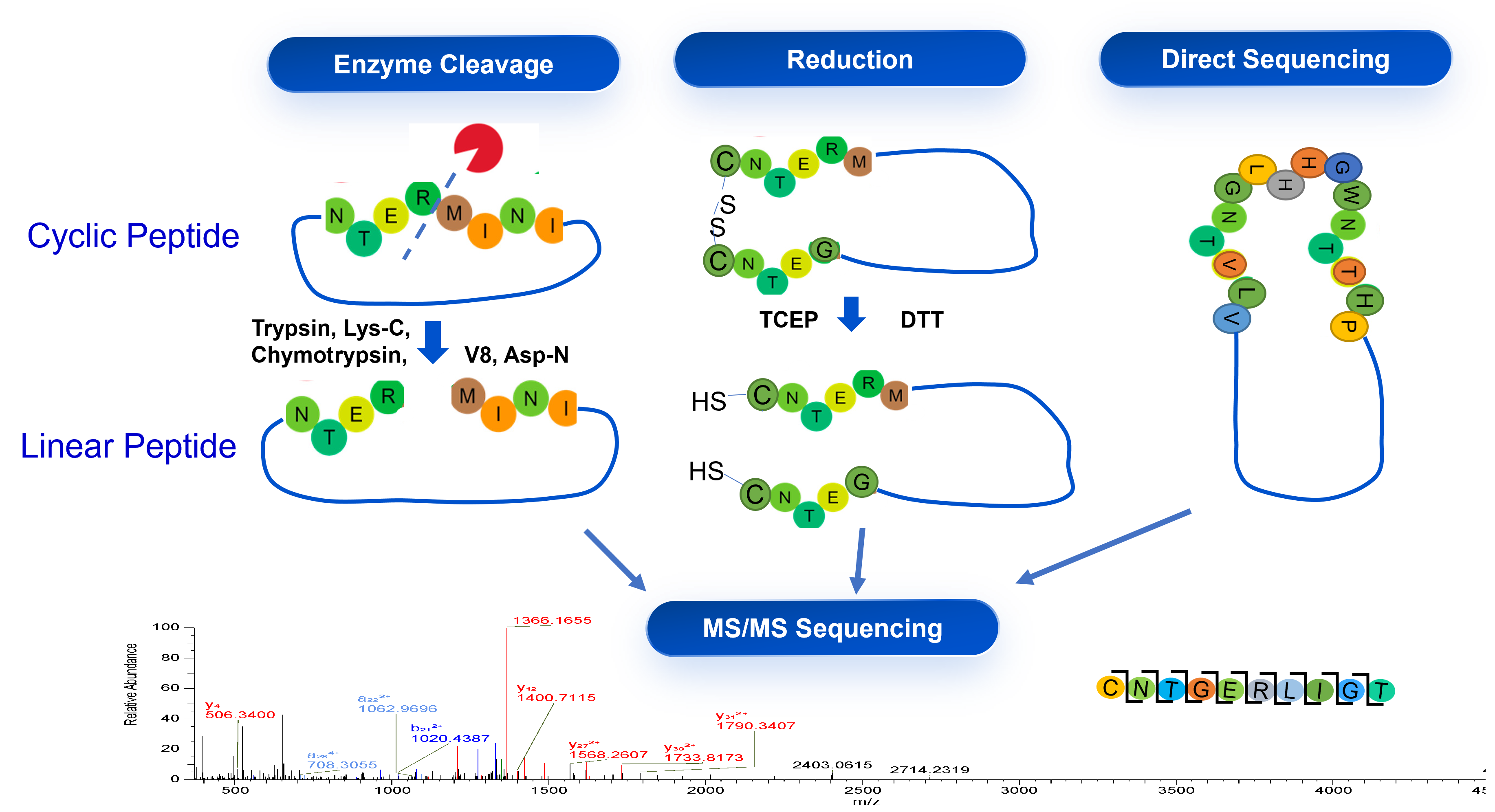
WuXi TIDES developed the method for the sequencing of peptides with more complex structures comparing to linear peptides for the identification and release of peptide drug substance.
Case Study 3: Chiral Amino Acid Analysis
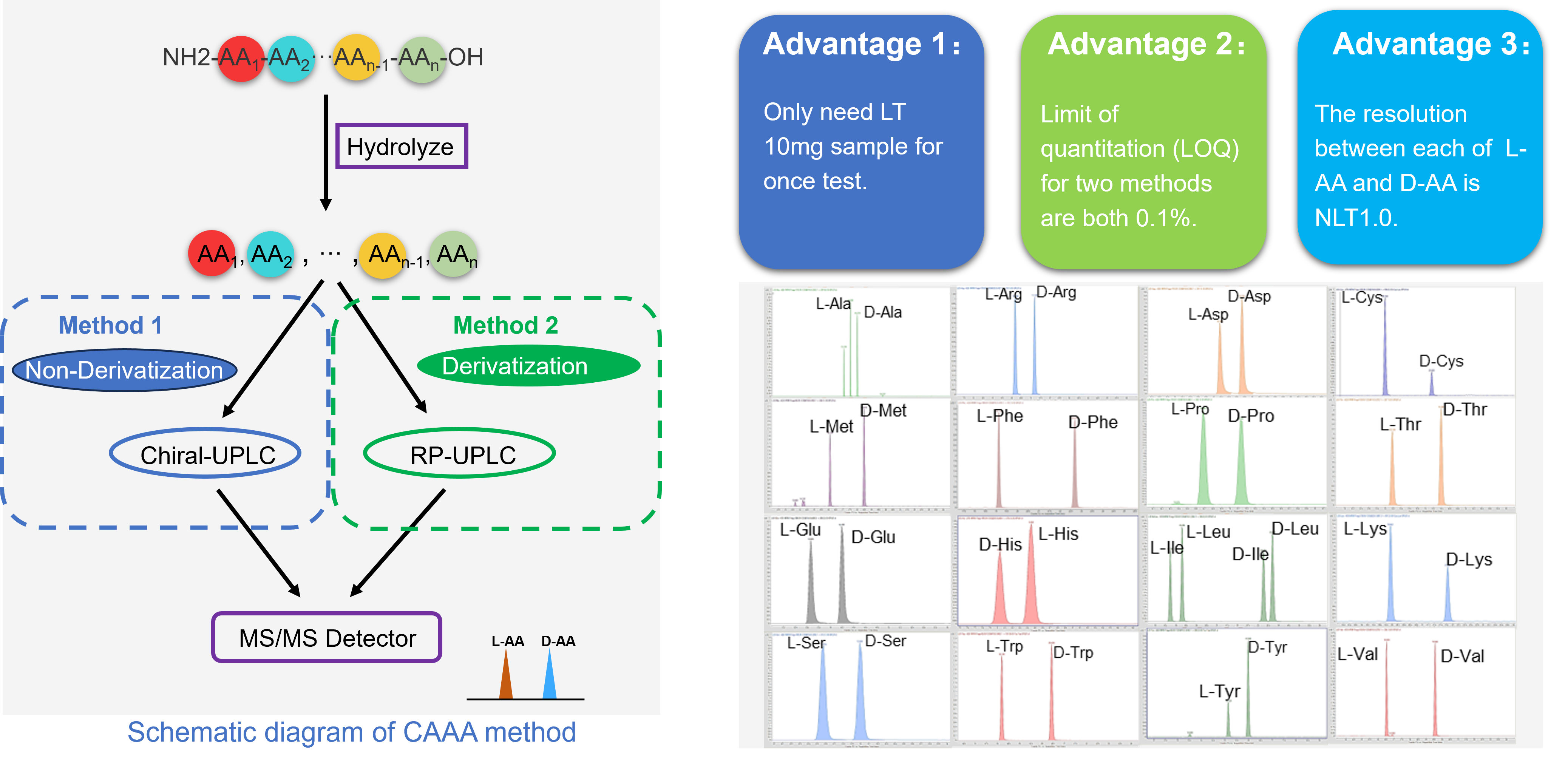
Accurately determining the enantiomeric purity of peptides can pose significant challenges. Peptides with different amino acid isomers share identical physicochemical properties, making intact analysis difficult. Free amino acids do not retain well for HPLC-UV analysis, and peptides containing multiple amino acids can interfere with each other during the analysis. Despite these challenges, we possess the capability to analyze the enantiomeric purity of all 19 isomeric natural amino acids (Asparagine and Aspartic acid, Glutamine and Glutamic acid are reported together), as well as Ornithine.





