Oligonucleotide API Service
- Select
- Select
Our oligonucleotide API process development and manufacturing platform offers comprehensive services, from preclinical to commercial stages. We’ve established industry-leading capabilities and capacities to support your needs across all development stages, ensuring knowledge retention throughout the product lifecycle. Moreover, our drug product site in Wuxi City, China is with 1-hour drive from the API site in Changzhou, China, enabling a faster, easier, and more cost-effective oligonucleotide CMC development for both API and drug product.
We support various oligonucleotide modalities, including but not limited to:
- DNA | ASO | PMO
- miRNA | siRNA | sgRNA
- Aptamer
- Various Sugar / Backbone Modified Oligonucleotides
Process Development
We have a highly experienced oligonucleotide R&D team comprised of several hundred scientists.
- Cover a broad range of molecule types, including ASO, siRNA, aptamer, oligonucleotide conjugates, PMO, and PPMO
- Utilize a variety of modified monomers in our processes (2'-H, 2'-OH, 2'-F, 2'-OMe, 2'-OMOE, LNA, cEt, LNA, Spacer,…)
- Specialize in the synthesis of chiral oligonucleotides
- Capable of modifying the 3'-end and/or 5'-end of molecules for a variety of compounds, including GalNAc, triphosphate, cholesterol, saccharides, peptides, and maleimide, among others
Manufacturing
We provide reliable and robust supplies of your oligonucleotide APIs, ranging from millimoles to moles. Our unique CRDMO platform ensures knowledge retention and seamless scale-up throughout your product’s life cycle. Furthermore, we expedite your development by streamlining cross-functional teams during different stages including API, drug product, analytical and regulatory dossier preparation.
We operate approximately 30 oligonucleotide manufacturing lines at various scales, including 4 large-scale lines (OligoProcess™) at our site in Changzhou, China. Additionally, we are planning to add more lines at Taixing & Singapore site.
Experience Highlights: For a commercial manufacturing project, we successfully completed 100 batches of 900 mmol DNA production. This included PPQ enabling studies and PPQ campaigns, resulting in the delivery of more than 400 kg of product within a span of 9 months.
Case Study: Accelerate Commercial Manufacturing of an Oligonucleotide with WuXi Speed.
Changzhou Site, China
Taixing Site, China
Key Equipment
Synthesis
- OligoPilot™100
- OligoPilot™400
- OligoPilot™2000
- OligoProcess™
- 5-100L PMO Synthesizers
Purification
- Hipersep® M450
- Hipersep® Pilot
- ÄKTAProcess™
- ÄKTA BioProcess™
UF / DF / TFE
- UniFlux™10
- UniFlux™30
- UniFlux™120
- Thin Film Evaporator
Lyophilization
- Tray Lyophilizers (0.5 m2 - 20 m2)
Access to New Technologies
Traditional oligonucleotide development and manufacturing processes often face sustainability challenges, including high waste, poor atom economy, and low energy efficiency. At WuXi TIDES, we provide our global customers with open access to innovative technologies for oligonucleotide development and manufacturing. These technologies aim to foster a greener, more efficient, and cost-effective process.
Liquid Phase Oligonucleotide Synthesis
Biocatalysis
Thin Film Evaporation
Spray Dried Dispersion
API in Solution
Continuous Purification
Liquid Phase Oligo Synthesis
Biocatalysis
Thin Film Evaporator
Spray Dried Dispersion
Isolate API Solution
Continuous Purification
Phosphorodiamidate Morpholino Oligomers (PMO)
Phosphorodiamidate Morpholino Oligomers (PMO) are a type of oligonucleotide molecule. These short, single-stranded DNA analogs are built upon a backbone of morpholine rings connected by phosphorodiamidate linkages. Although they are routinely used for gene silencing and have been developed into excellent antisense reagents in PMO-based drugs, synthesizing PMO remains a challenging task.
At WuXi TIDES, we have developed a high loading solid-phase PMO synthesis process. This process achieves over 50% yield, over 75% crude purity, and more than 90% final product purity with less than 0.045 EU/mg endotoxin. Our chemistry know-how allows us to make various PMO 5′ modifications with an improved cleavage & de-protection process.
To support large-scale production, we have customized PMO synthesis reactors up to 100L. Our team has successfully completed PMO development and manufacturing projects on a kilogram scale.

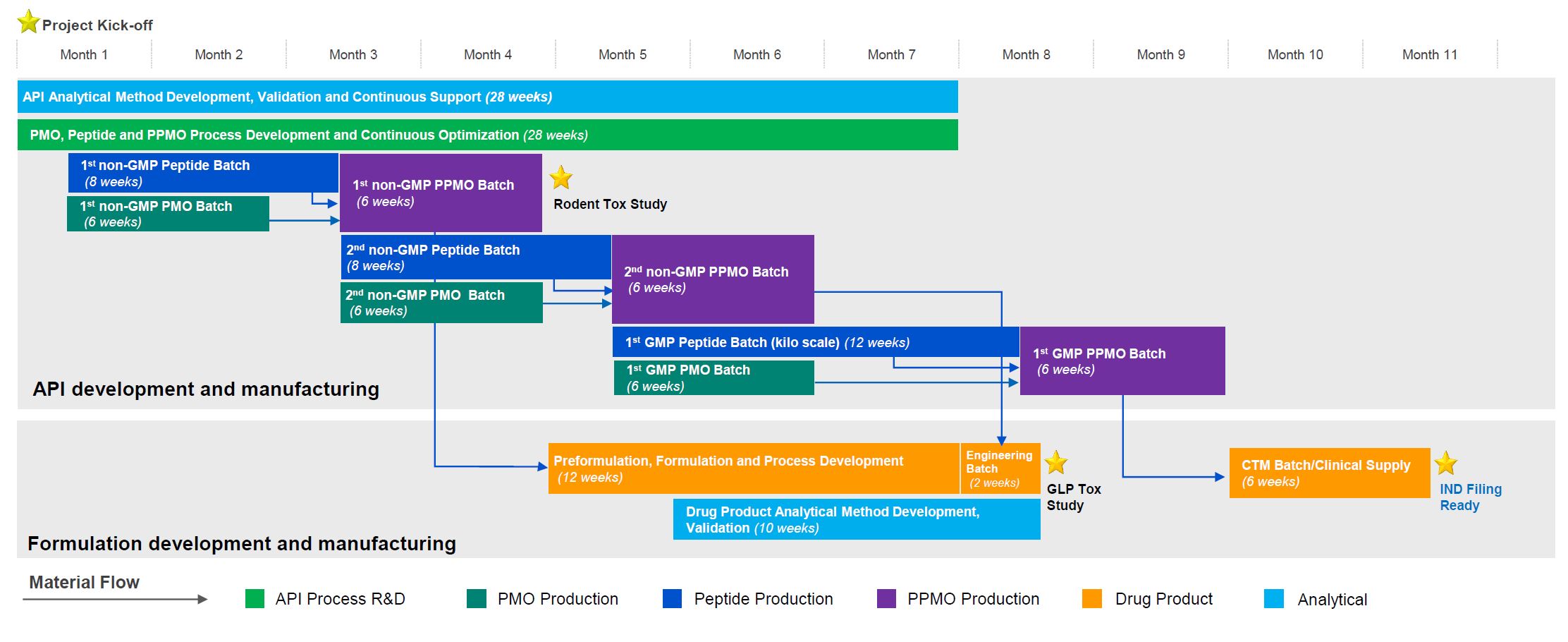
Our oligonucleotide and peptide API platforms are both located at our site in Changzhou, China. This strategic location is well-suited for the development and manufacturing of oligonucleotide and peptide conjugates, especially Peptide-PMO (PPMO). To date, our team has successfully completed dozens of PPMO projects. The following case study illustrates how our cross-functional teams seamlessly collaborate with in-parallel activities, providing a fast, efficient, and flexible solution for PPMO development. Learn more from our case study video here.
Case Study: IND Ready in 11 Months for a PPMO Drug Candidate
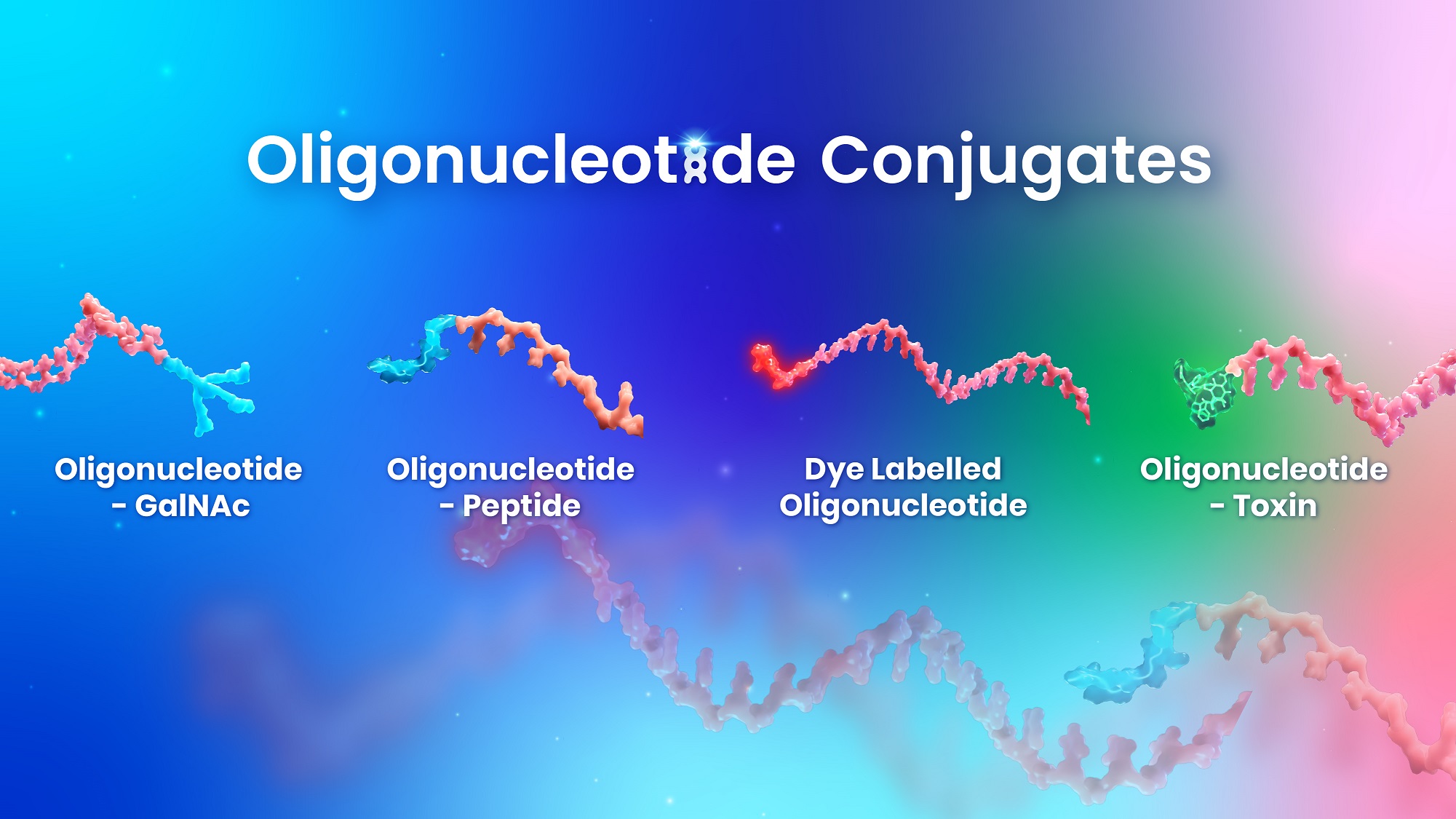
Oligonucleotide Conjugate
When your oligonucleotide conjugate drugs enter the preclinical phase, you may encounter challenges if your synthesis methodology or conjugation techniques aren’t suitable for scale-up or first-in-human needs.
WuXi TIDES is well positioned to provide a one-stop solution for complex oligonucleotide-base conjugated drugs, from discovery through development to commercial manufacturing. Our strong capabilities extend to oligonucleotides and small molecules, including ligands, toxins, linkers, lipids, PEG, and more. With readily scalable facilities, we offer integrated CMC services and highly reliable solutions for developing and manufacturing oligonucleotide APIs and all common ligands. We also provide conjugation chemistry to streamline your projects from preclinical studies to clinical trials. Our dedicated teams significantly reduce the time required for CTM batches and simplify material transfers.
Ligands
- Peptide
- Lipid
- GalNAc
- Cholesterol
- PEG
Linker Chemistry
- Amide Coupling
- Thiol-maleimide Addition
- Click Chemistry (CuAAC | SPAAC)
- Broad Selection of Spacers
- Custom-tailored Linkers
Topology
- 3'- or 5'-conjugation
- Complex Dual Conjugation: 3’,5’- | 3’,3’- | 5’,5’-
- Multi-valency: mono- | di- | tri- | tetra
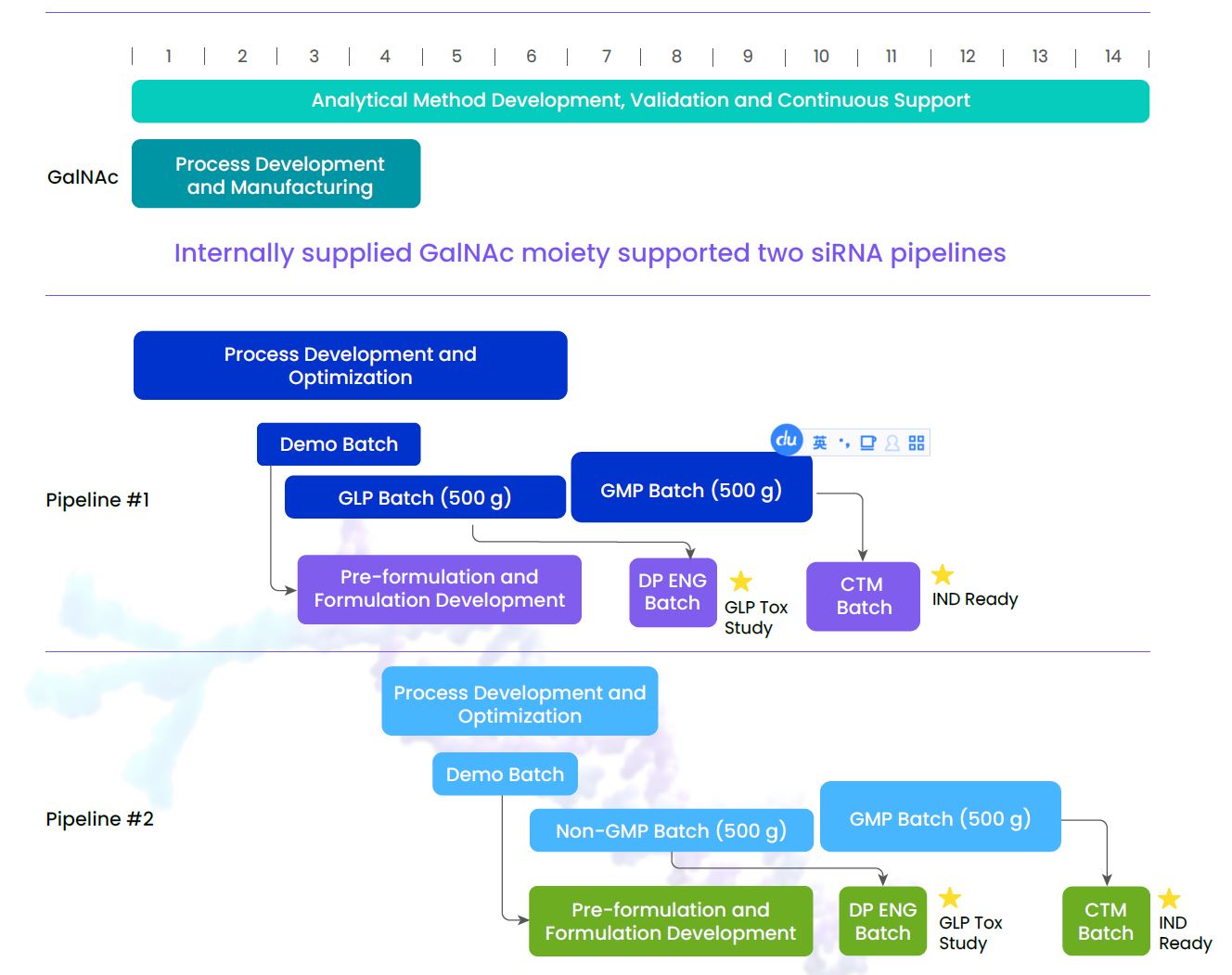
Case Study: Two siRNA IND CMC Packages Completed in 14 Months
A biotech company engaged WuXi TIDES to develop a siRNA-GalNAc drug candidate for cardiovascular disease. The original synthesis process for this 23-mer siRNA resulted in a yield of 13% and a crude purity of 18%. In addition, the GalNAc moiety was not commercially available. Our team’s objective was to accelerate this challenging molecule to IND filing as fast as possible. Utilizing innovative conjugation chemistry and tailored analytical studies, we significantly improved the crude purity and yield to 75% and 62%, respectively, delivering on this project in a timely and efficient manner. View the full article
Amidite Process Development & Manufacturing
Our team has extensive experience customizing over 1,000 amidite molecules, even those with the most challenging structures. This expertise supports your oligonucleotide projects from discovery through development to the commercial stage, including:
- Sugar Backbone (LNA, cEt, UNA, GNA, TNA amidite, etc.)
- Phosphorus Backbone (PACE, PS2, chiral amidite )
- 2'/3'/5' Modified Amidite
- Base Modified Amidite (tRNA base, deaza base)
In additon to discovery synthesis, we offer amidite process development and manufacturing services, with dedicated plants specifically for amidite production. Besides, we established amidite impurity control strategy by systematically conducting assessment of amidite impurities and their impact on oligonucleotide API quality. During the process, 1,000+ amidite impurities were synthesized.
Among 500+ catalog amidites, we also provide 40+ amidite products available at commercial scale, including DNA, 2’-MOE, 2’-OMe, 2’-F, LNA, PMO monomer, etc. Selected common amidite DMFs available. All our commercial amidite products are supported by comprehensive Drug Master Files (DMF) to ensure they meet regulatory requirements for use in IND and NDA as regulatory starting materials for oligonucleotide manufacturing.
Sample Amidite Molecules
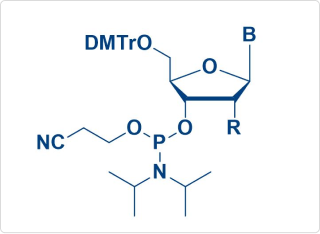
RNAi-Amidite
(2′-F, OMe Moe, OTBS, etc.)
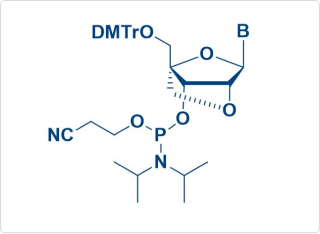
LNA Amidite
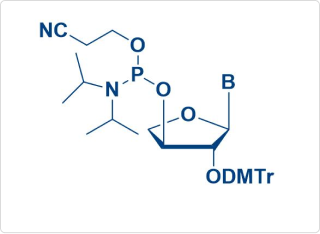
TNA Amidite
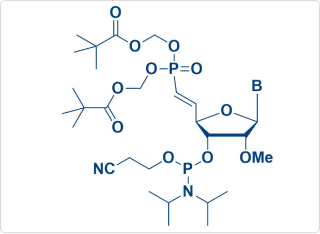
Vpm-POM
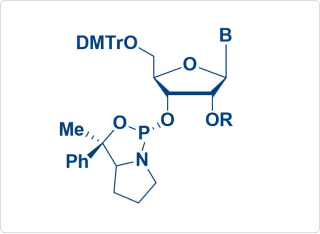
Chiral Amidite
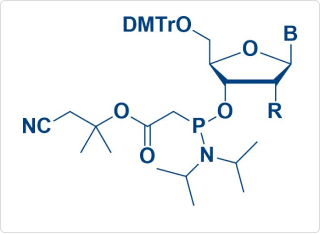
PACE Amidite
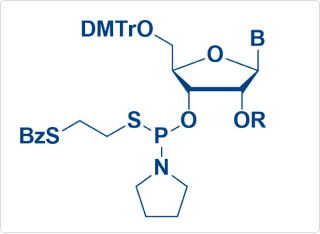
Thio-Amidite
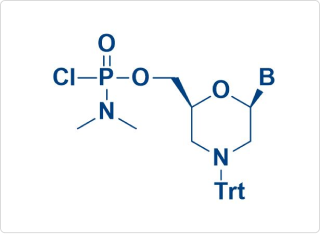
PMO Monomer
GalNAc Process Development & Manufacturing
Our teams have extensive experience in the production of various types of GalNAc molecules, including Mono-GalNAc, Tri-GalNAc, Tetra-GalNAc, GalNAc amidite, GalNAc PFP ester, GalNAc N3, and GalNAc-PEG conjugates, among others. 100+ GalNAc compounds were synthesized
We offer GalNAc custom synthesis, process development, and manufacturing support from the discovery phase through to commercial launch. Our batch scales range from milligrams to over 50 kilograms.
GalNAc development and manufacturing services are provided at our Changzhou site, China. We successfully optimized GalNAc resin loading process, increasing SPOS yield by 50%.
With various GalNAc building blocks and CPG/PS conjugate resins, we are well-equipped to accelerate your GalNAc-oligonucleotide projects.
Related Resources

The Role of Benchmarking Critical Impurity Control in Therapeutic Oligonucleotide Phosphoramidites







