Oligonucleotide Analytical
Home - Services & Solutions - Oligonucleotide - Development & Manufacturing - Analytical
WuXi TIDES has a dedicated analytical team with extensive experience and expertise in oligonucleotide analysis across all development phases from pre-clinical development to commercial manufacturing, covering both drug substance and drug product.
Equipped with state-of-the-art instruments and co-located with the API process development team, our oligonucleotide analytical service streamlines your oligonucleotide development process with a customized, flexible, and integrated analytical solution.
Analytical Development
Drug Substance Specific Analytical Services
- Analytical development for drug substance specific methods (purity, sequencing) and feasibility for oligonucleotide generic methods
- Forced degradation study with impurity qualification and quantitation
- Release testing and stability study under R&D conditions
- Reference standard and impurity marker qualification and retesting
- Physicochemical property study and structure elucidation
- Microbiological safety: endotoxin limit and quantitation testing, bioburden for microbial limit test, microbe ID and strain typing
Drug Product Specific Analytical Services
- Container-Closure Integrity Testing (CCIT), including quantitative testing such as vacuum decay method, high-voltage leak detection and qualitative testing such as microbial challenges, tracer liquid, etc.
- Foreign and particulate matter observe in situ, verifying by flow imaging (MFI) and identifying via IR/Raman/EDX, osmolality
- Purity by LC-MS, including ID and quantitation utilizing Q-TOF HRMS and quadrupole LC-MS specifically for hard-to-resolve parenteral impurity analysis
- Sterility and microorganism identification

Quality Control
- Method qualification and validation (IND/NDA, commercial)
- Transfer GMP validated methods according to USP <1224>
- Full release testing (raw materials, reagents, intermediates, drug substance and drug product)
- Accelerated and long-term stability studies under ICH compliance
Special Capabilities
- Process related impurity identification: 2D UPLC coupled with Q-TOF HRMS
- Identification by tandem LC-MS/MS for sequence confirmation
- Oligonucleotide backbone composition by 31P NMR
- Diastereomer fingerprinting by 31P NMR


Key Equipment
- Chromatography: UPLC, HPLC, GC, IC, 2D-UPLC, HPLC-CAD
- Mass Spectrometry: QTOF, MALDI-TOF, LC-Orbitrap, LC-MS, LC-QQQ, 2D LC-MS, GC-MS
- Physicochemical Testing: FT-IR, UV-Vis XRPD, DVS, DSC, TGA, polarimetry, 400/600 MHz NMR, MEC, Tm, CD
- Elemental Analysis: ICP-OES, ICP-MS, elemental analysis (CHN)
Case Study 1: Purity and Impurities by LC-UV-HRMS (tRNA/gRNA)
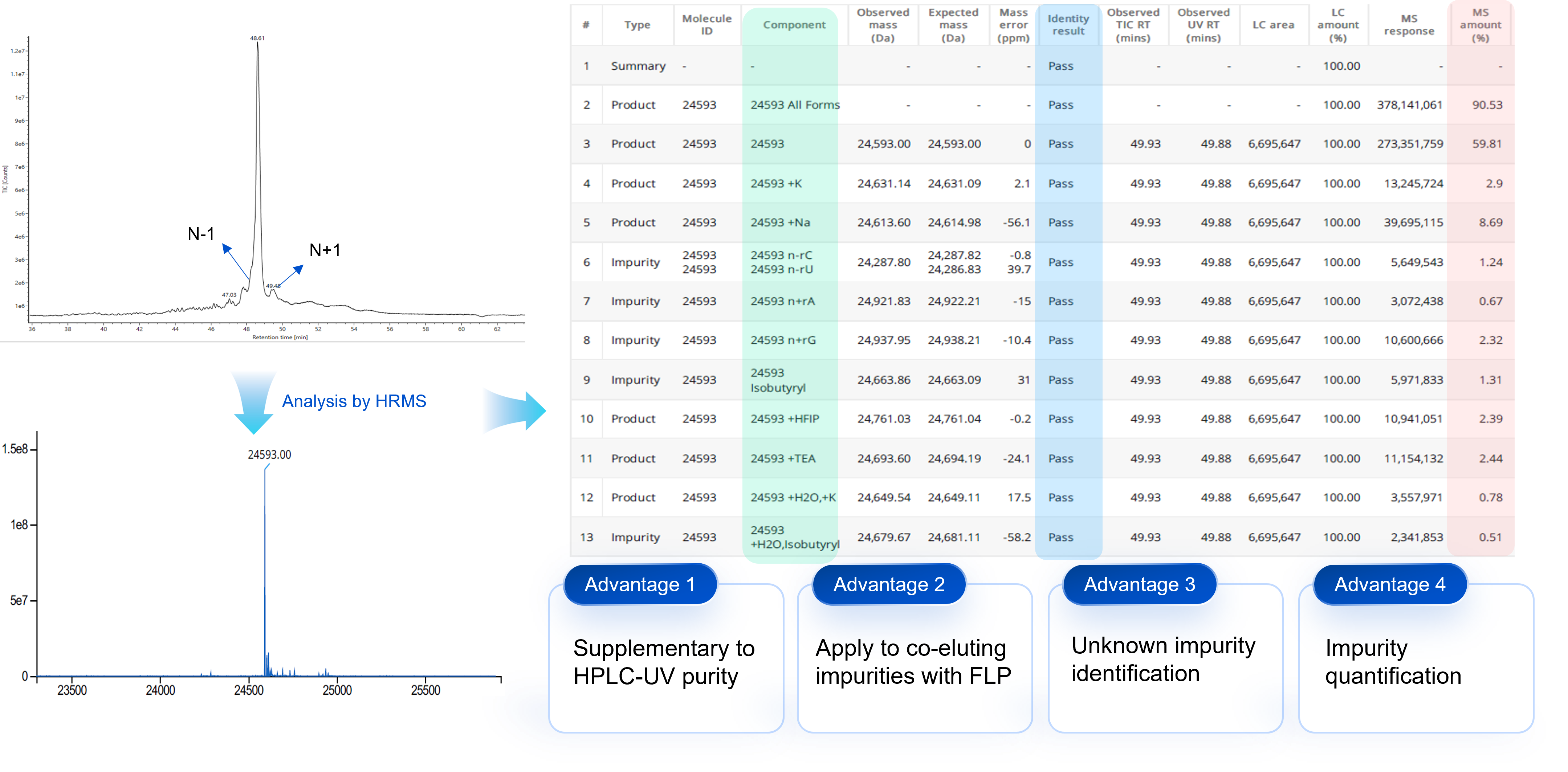
For the impurites that are hard to be separated from or co-eluting with FLP in HPLC-UV, MS purity is utilized for the qualificaiton and quantitation of impurities in tRNA, sgRNA PMO/PPMO and ASO analysis.
Case Study 2: Purity and Impurities by LC-UV (sgRNA-100mer)
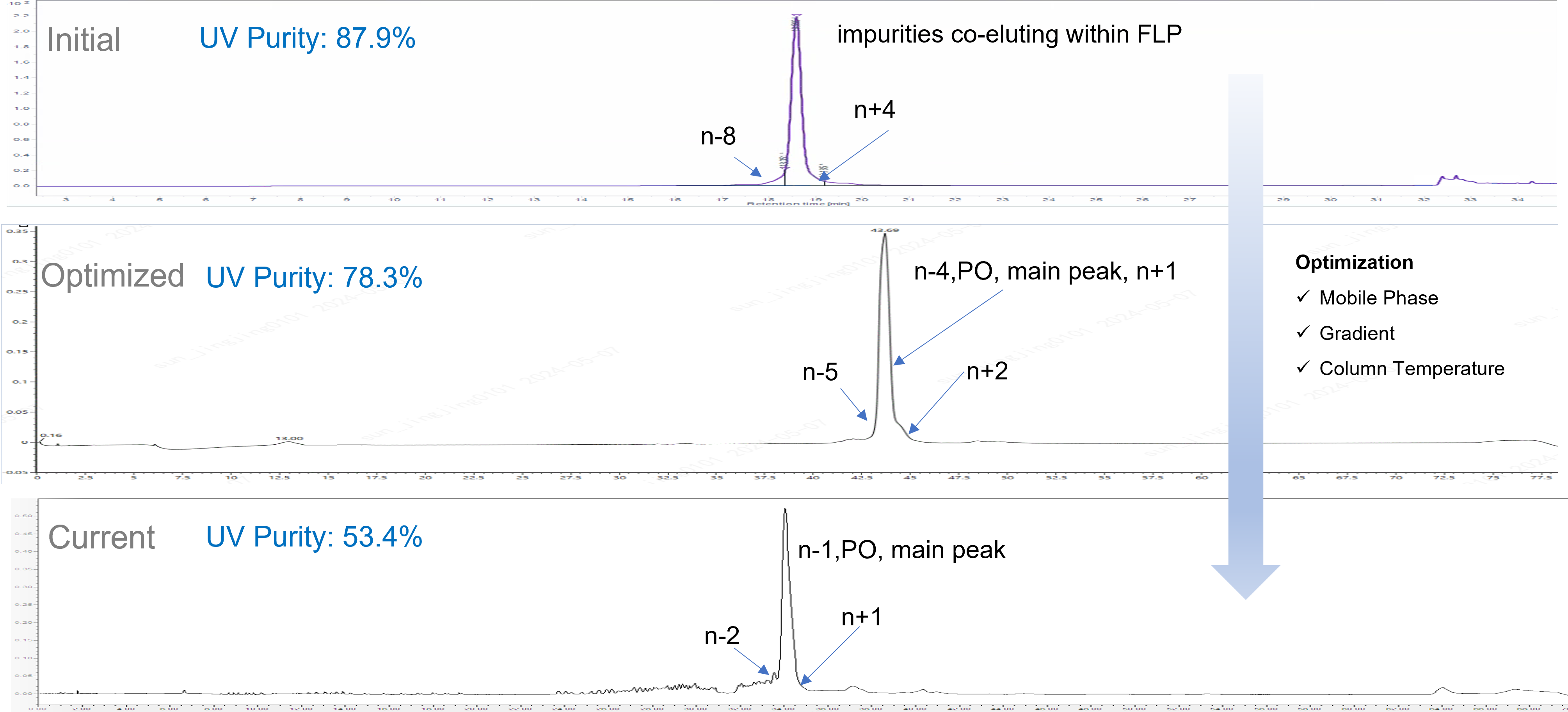
Evolution of resolution of HPLC purity method for sgRNA. From separation capability of n-8 and n+4 impurities initially to n-2 and n+1 impurities by current method at WuXi TIDES.
Case Study 3: Sequencing of PPMO
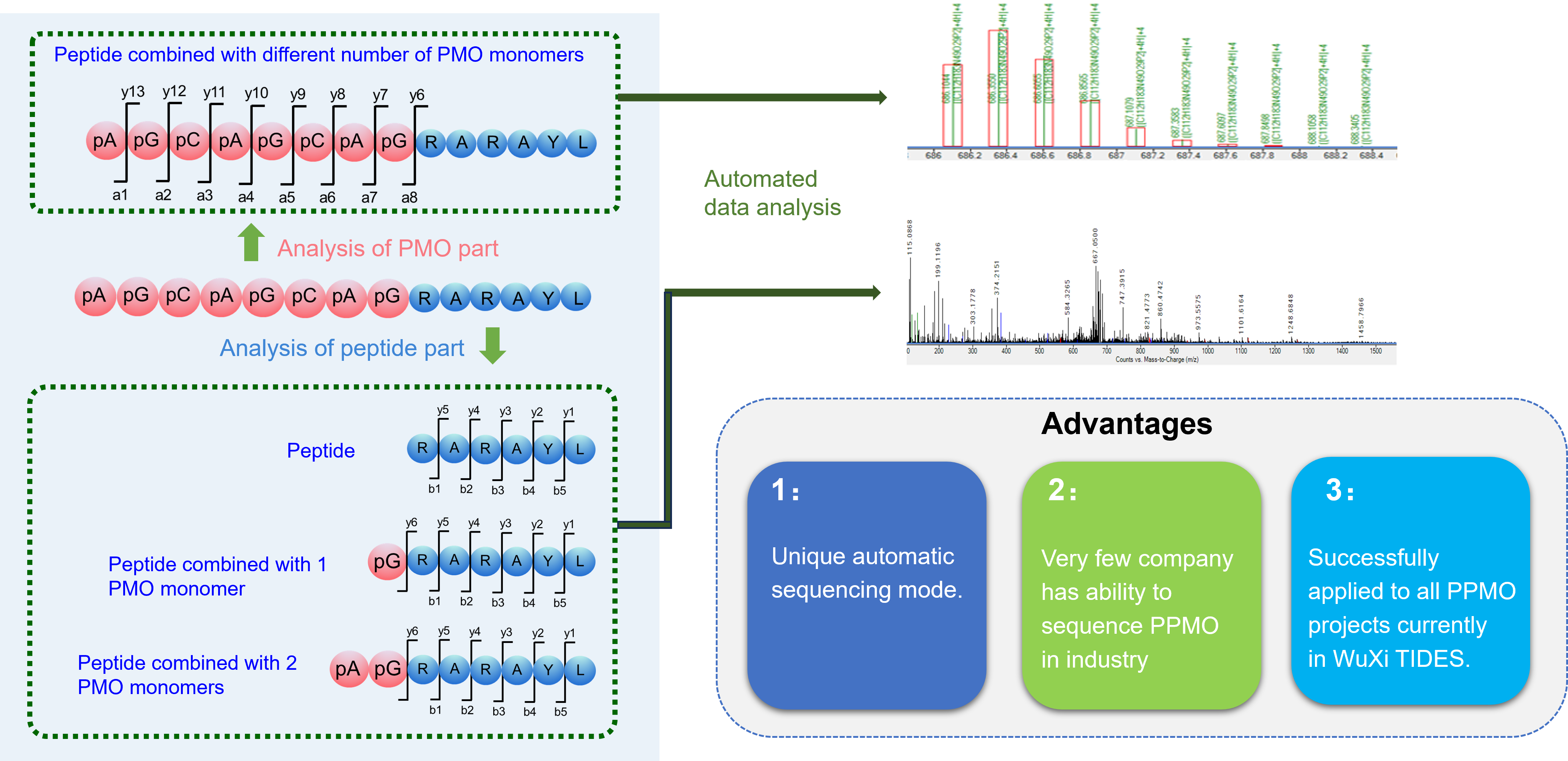
The determination of the Peptide-PMO sequence involves sequencing both the peptide and PMO components of the PPMO, as well as the conjugation process between the two intermediates.
Case Study 4: Systematically Screen Purity Methods for Improved Resolution
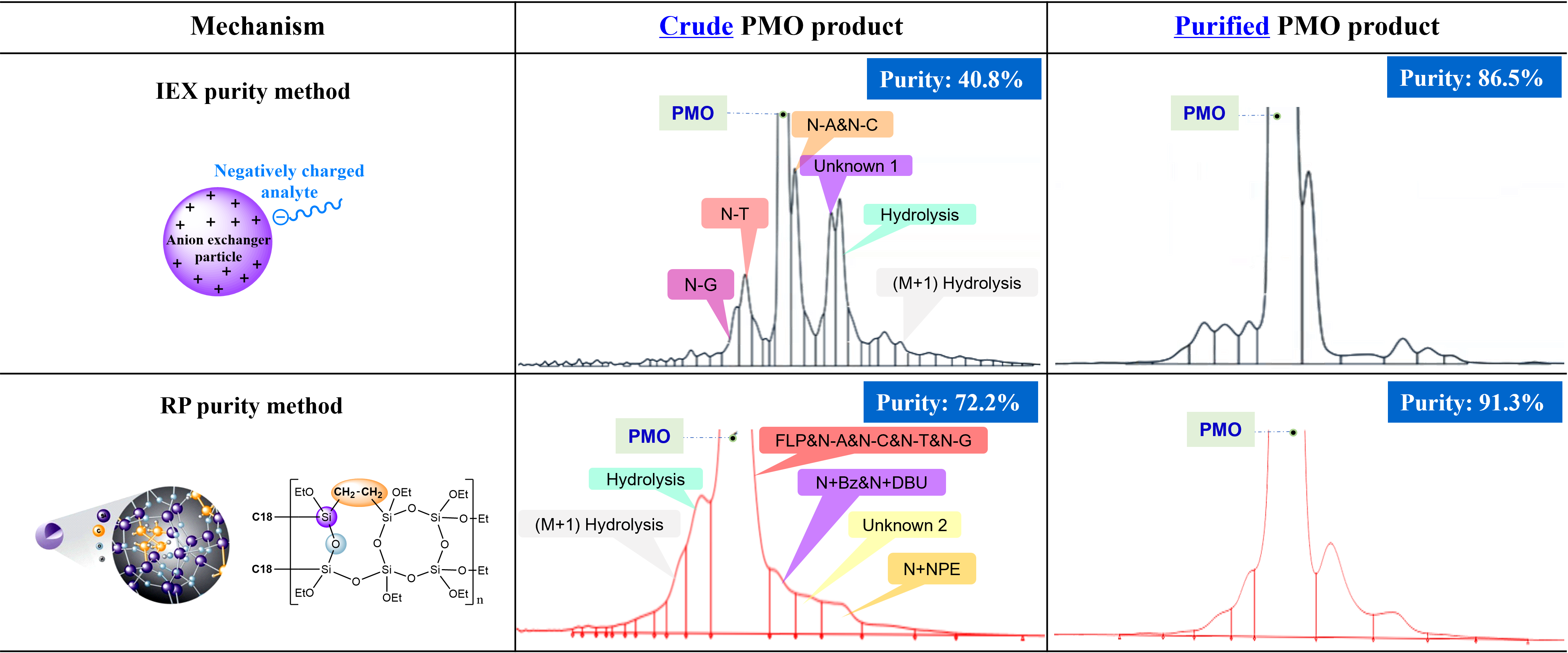
Both Ion-Exchange Chromatography (IEX) and Reverse-Phase HPLC (RP-HPLC) are utilized for the purity and impurity analysis of PMO product. These orthogonal chromatography techniques provide an accurate depiction of the product’s quality.
- WuXi STA has ability to screen methods systematically to get a better purity method with more resolved impurity peaks.
- IEX purity method and RP purity method display different impurity profiles.





