Monomer & Ligand
Home - Services & Solutions - Oligonucleotide - Discovery Synthesis - Monomer & Ligand
Amidite Synthesis
Our team has extensive experience with 1,000+ customized amidite molecules with most challenging structures, to support your oligonucleotide discovery projects. Additionally, we offer 500+ amidite catalog products readily available.
Visit Amidite Catalog e-Shop
We are experienced in various modifications:
- 2’/3’/4’ modification with GalNAc moiety, lipid, amine, alkyne
- Sugar Skeleton Modification: bicyclic sugar, aza/thio sugar, acyclic sugar
- Base Modification: 7-deaza A/G, Pseudo U (pU), N-Me pU
- Therapeutic RNA Modifications (2’-OMe, 2’-F, 2’-O-MOE)
- Phosphorus Backbone Modification: PACE, thiophosphoramidite, MeP, EtP
- Base Protecting Alternative (Bz, Ac, iBu, pac/iPr-pac, O-NPE, etc.)
- Functional / Conjugate Modifiers (phosphate reagents, amino & thiol linkers, spacers, cholesterol, dye, etc.)
Sample Amidite Molecules
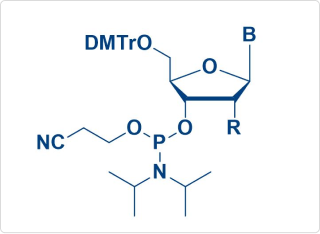
RNAi-Amidite
(2′-F, OMe Moe, OTBS, etc.)
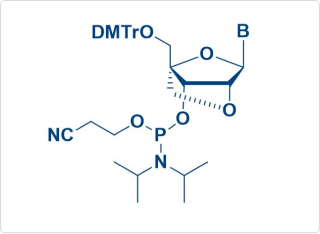
LNA Amidite
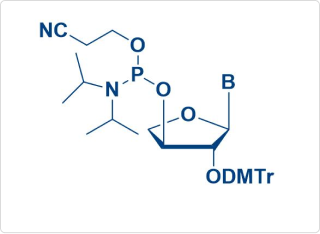
TNA Amidite
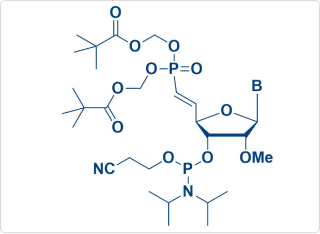
Vpm-POM
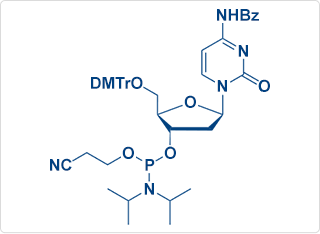
DNA C(Bz) Amidite
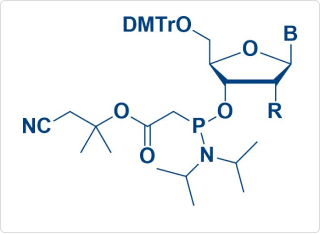
PACE Amidite
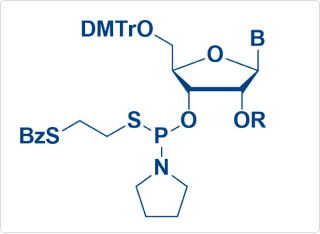
Thio-Amidite
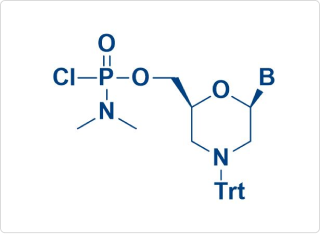
PMO Monomer
GalNAc Synthesis
N-Acetylgalactosamine (GalNAc) is an amino sugar derivative of galactose. Conjugation with GalNAc represents an efficient method for enhancing siRNA target organ accumulation and facilitating cellular uptake.
Our team has extensive experience synthesizing over 100 types of GalNAc molecules, including Mono-GalNAc, Tri-GalNAc, Tetra-GalNAc, GalNAc amidite, GalNAc PFP ester, GalNAc N3, and GalNAc-PEG conjugates, among others.
With more than 100 GalNAc building blocks and CPG/PS conjugate resins, we are well positioned to accelerate your GalNAc-oligonucleotide discovery projects.
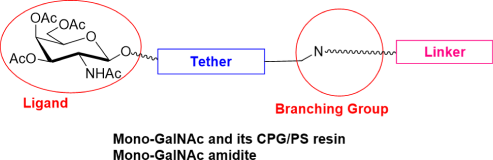
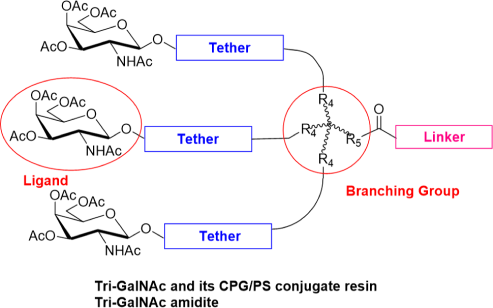
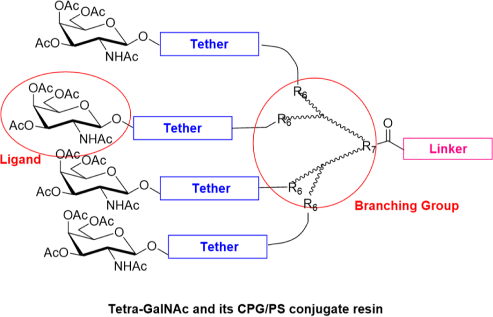
Overcome GalNAc synthesis challenges
There are many synthetic challenges for GalNAc because most of the intermediates are oily or foamy solids. The synthesis involves many columns and prep-HPLC purification steps, resulting scale-up difficulties and low yields. Analytical method for GalNAc synthesis is also challenging due to low UV response, complex impurity profile and poor stability.

Original Process
Oil Intermediates

Modified Process
Solid Intermediates
Enabled by strong chemistry platform, WuXi TIDES developed convergent routes through telescoping processes without column purification steps. The isolated intermediates were stable solids. We applied the precipitation process and/or spray drying process to reduce the needs for the columns and prep-HPLC steps. Our analytical teams identified many GalNAc-related impurities to support method development and release testing.





