WuXi TIDES 拥有⼀⽀专业的多肽分析团队,具有丰富的经验和专业知识,覆盖从临床前开发到商业化生产的所有阶段,包括原料药和制剂。
我们的多肽分析平台配备了尖端的仪器,并与原料药工艺开发团队位于同⼀基地,为您的多肽开发提供定制化、灵活且一体化的分析解决方案,从而简化开发流程。
分析开发
原料药分析服务
- 多肽原料药专属的分析方法开发(纯度、测序)和通用方法的适用性研究
- 强制降解研究,杂质鉴定和定量
- 研发条件下的放行试验和稳定性研究
- 标准品和杂质对照品的标化和复测
- 理化性质研究及结构解析
- 微生物安全: 内毒素限度和定量测试、生物负载和微生物限度测试、微生物菌株鉴定
制剂分析服务
- 容器封闭完整性测试(CCIT),包括真空衰减法、高压泄漏检测等定量测试以及微生物挑战、示踪液等定性测试。
- 可见异物和颗粒物检查,通过流动成像 (MFI) 确认,并通过 IR/Raman/EDX 、渗透压进行识别
- 通过 LC-MS 测定纯度,包括利用 Q-TOF HRMS 和四极杆 LC-MS对较难分析的注射剂杂质进行鉴别和定量

Peptide Enantiomeric Purity Test
Determining the enantiomeric purity of peptides accurately can be challenging. Peptides with different amino acid isomers share the same physicochemical properties, making them difficult to analyze intact. Free amino acids do not retain well for HPLC-UV analysis, and peptides containing multiple amino acids can interfere with each other during analysis. Despite these challenges, we have the capability to analyze the enantiomeric purity of all 19 isomeric natural amino acids (Asparagine and Aspartic acid, Glutamine and Glutamic acid are reported together), as well as Ornithine.

质量控制
- 方法确认和验证(IND/NDA 、商业)
- 根据USP <1224> 对经过GMP验证的方法进行正式转移
- 工艺流程全覆盖的放行检测(原材料、试剂、中间体、原料药和制剂成品)
- 符合 ICH 要求的加速和长期稳定性研究
特殊能力
- 工艺相关杂质鉴别:二维高效液相色谱结合QTOF高分辨质谱
- 通过串联LC-MS/MS 进⾏序列确认
- 氨基酸分析及手性氨基酸纯度分析:多肽产品酸解衍生化后,进行LC-MS分析,准确评估多肽序列中各种氨基酸的比例及D-异构体杂质的含量
- 肽图鉴别:酶解后进行液相色谱-质谱分析,对多肽原料药进行表征和确认

Other Equipment
- Amino Acid Analysis (AAA)
- Elemental Analysis (CHN)
- Enantiomeric Purity
- Polarimetry
- Refractometry
- Titration
- Turbidimetry
- Osmolality
- Total Organic Carbon (TOC)
- Total Viable Aerobic Counts
- Endotoxins (LAL-test)
- Heavy Metals
- Sulfated Ash
- Residue on Ignition
- Polydispersity

关键设备
- 色谱:UPLC、 HPLC、GC 、IC 、2D-UPLC、 HPLC-CAD
- 质谱:QTOF、 MALDI-TOF、 LC-Orbitrap、 LC-MS、 LC-QQQ、2D LC-MS、GC-MS
- 理化性质测试:FT-IR, UV-Vis XRPD, DVS, DSC, TGA, polarimetry, 400/600 MHz NMR, MEC, Tm, CD
- 元素分析:ICP-OES, ICP-MS, elemental analysis (CHN)
Case Study 1: Assessing Peptide Main Peak Purity by 2D-UPLC-TOF
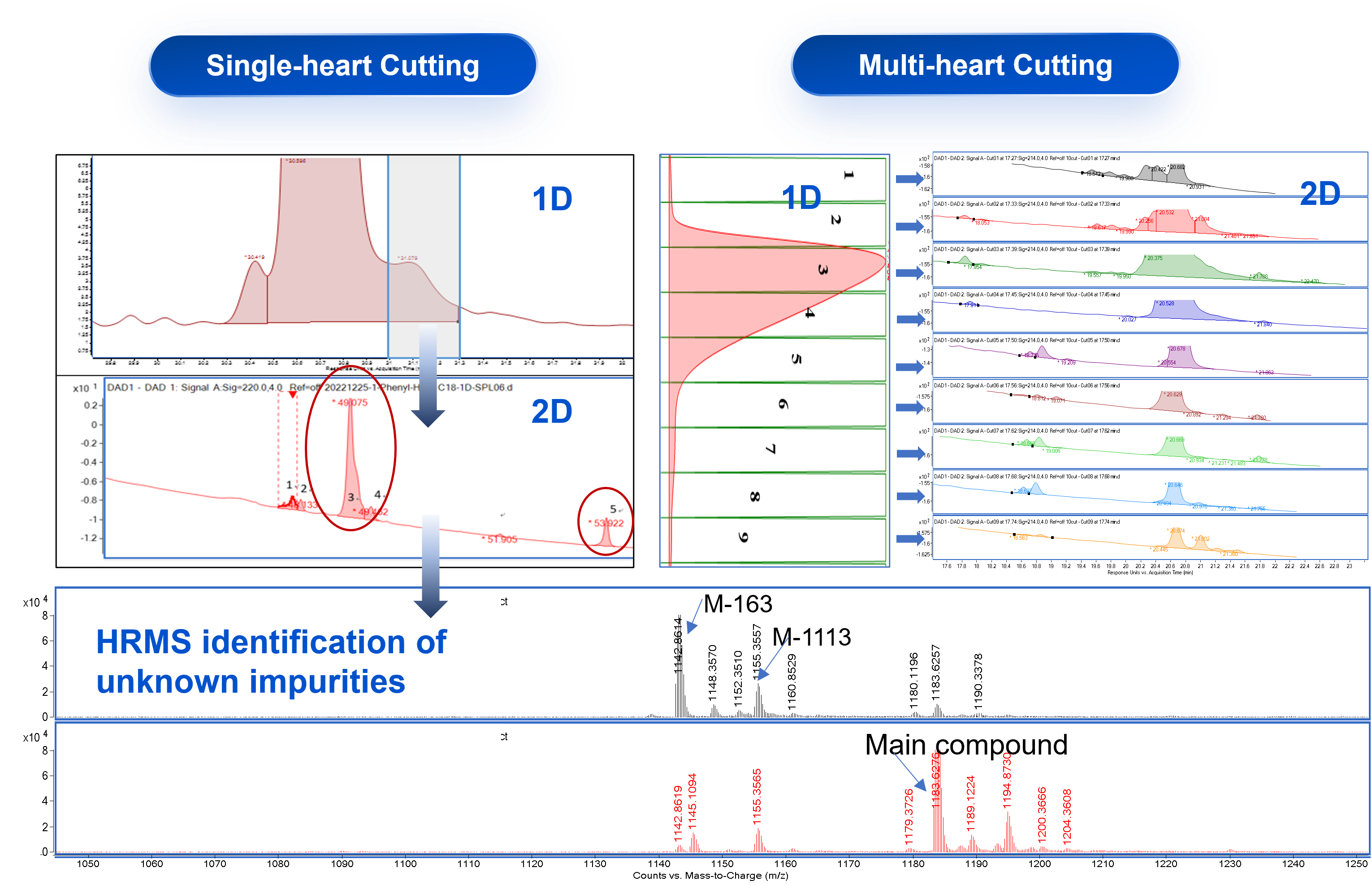
Multi-heart cutting 2D-UPLC-TOF is routinely used for the quantitation and qualificaiton of impurites that prove challenging to be separated by regular RP-HPLC, which greatly increases the efficiency for the impurity analysis and accuracy of data.
Case Study 2: Sequencing of Cyclic Peptide
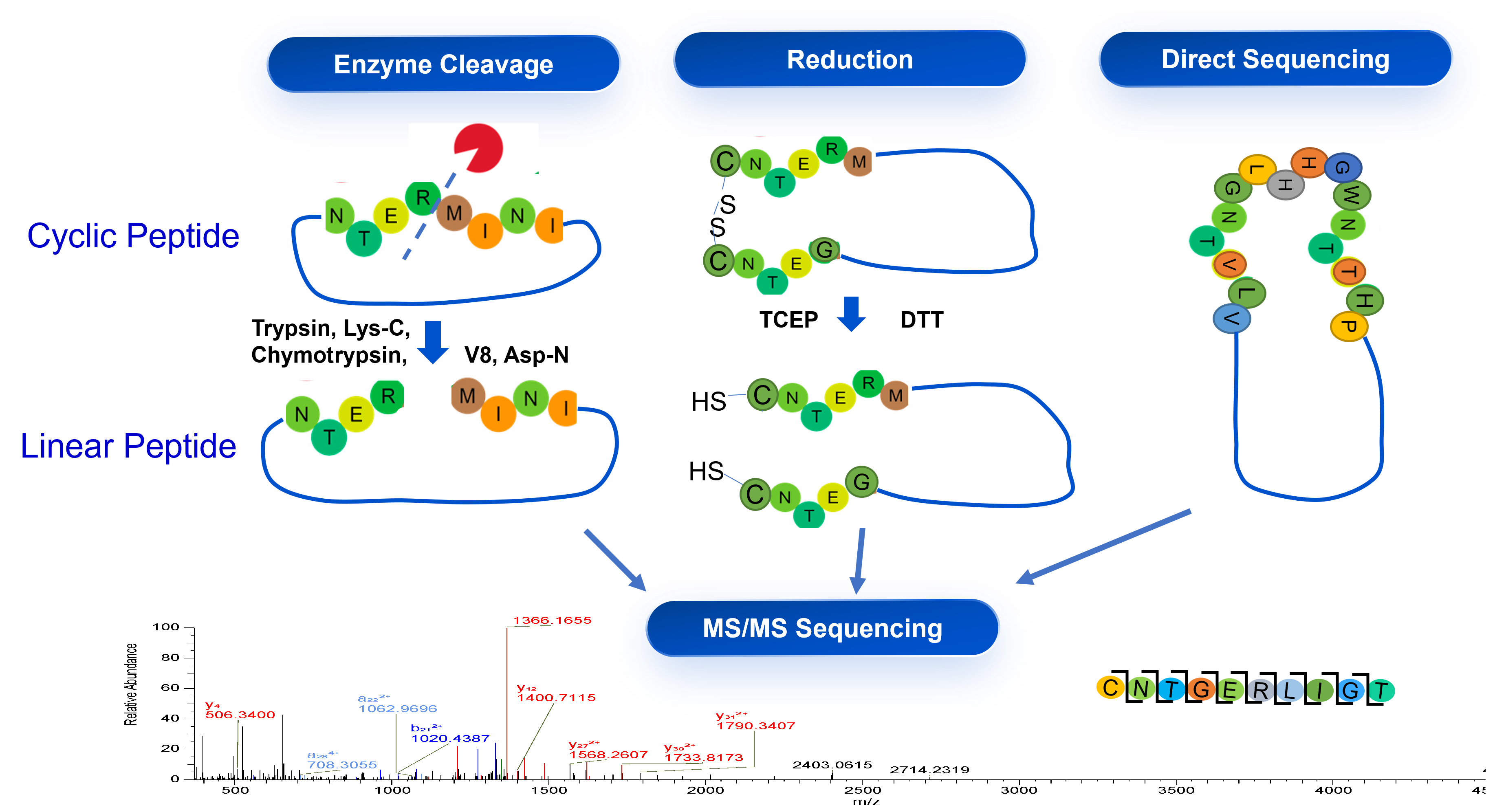
WuXi TIDES developed the method for the sequencing of peptides with more complex structures comparing to linear peptides for the identification and release of peptide drug substance.
Case Study 3: Chiral Amino Acid Analysis
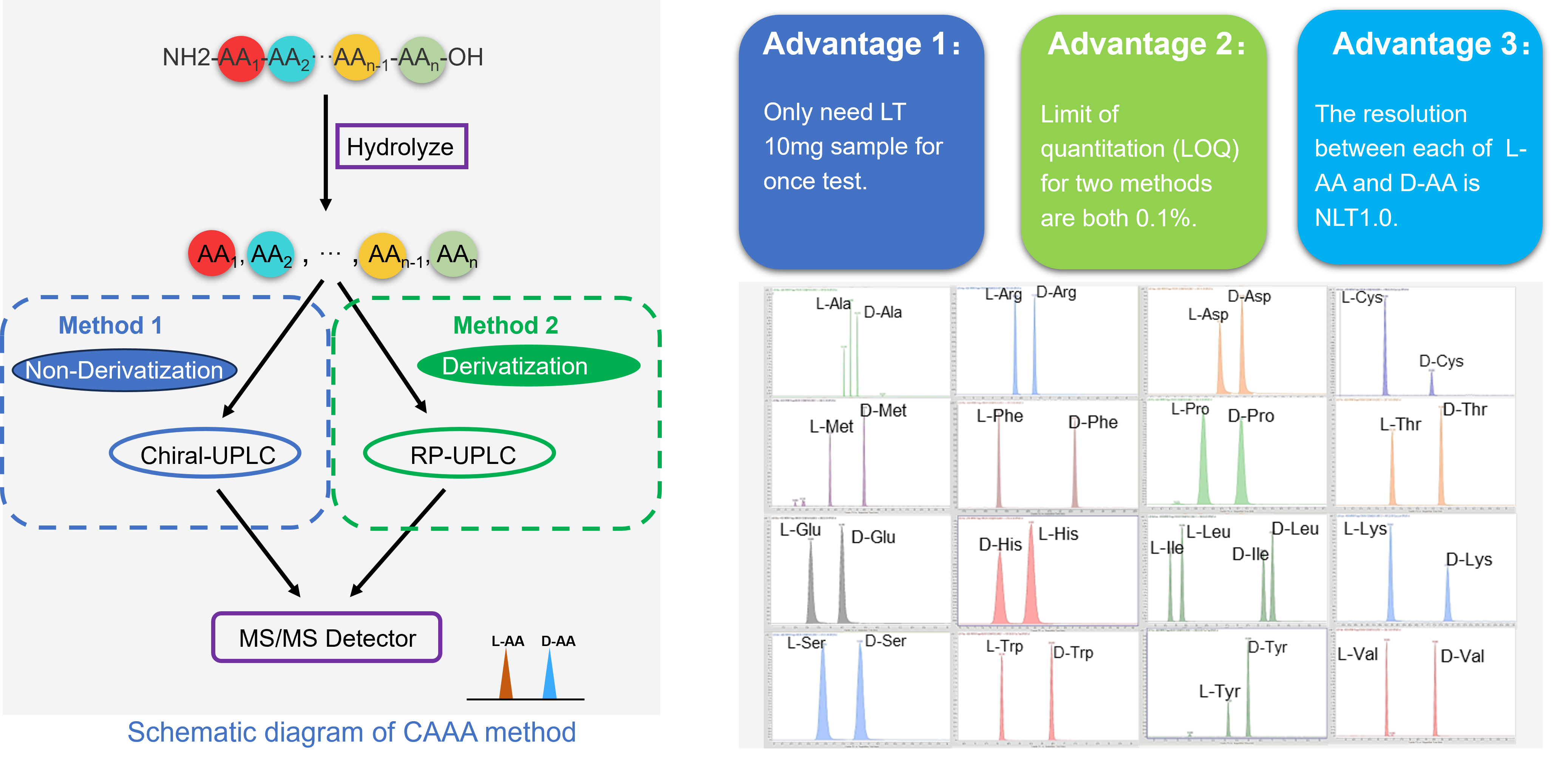
Accurately determining the enantiomeric purity of peptides can pose significant challenges. Peptides with different amino acid isomers share identical physicochemical properties, making intact analysis difficult. Free amino acids do not retain well for HPLC-UV analysis, and peptides containing multiple amino acids can interfere with each other during the analysis. Despite these challenges, we possess the capability to analyze the enantiomeric purity of all 19 isomeric natural amino acids (Asparagine and Aspartic acid, Glutamine and Glutamic acid are reported together), as well as Ornithine.




